
Pregnancy puts pressure on the legs. Your buyers need support that feels medical, looks modern, and fits more body types, including plus-size mothers. That’s where OEM maternity compression socks come in.
OEM maternity compression socks are no longer a niche SKU. In 2024, the maternity compression socks market reached about $420–535 million and is set to grow at around 7.1–7.2% CAGR to 2033. For brands, retailers, and Amazon sellers, that growth means one thing: if you sell to pregnant women, you need a focused OEM strategy, not a generic compression sock supplier.
Maternity buyers want safe compression, soft fabrics, fashion options, and sizes that actually fit, especially in plus-size and wide-calf. You want stable margins and low product risk. This article bridges both sides.
Why do pregnant women need maternity compression socks?
Pregnancy increases the risk of swelling, varicose veins, and deep vein thrombosis (DVT). OEM maternity compression socks apply graded pressure from ankle to calf, which supports blood flow and helps manage pain and edema. Medical studies support daily use, especially in the second and third trimesters, under professional guidance.
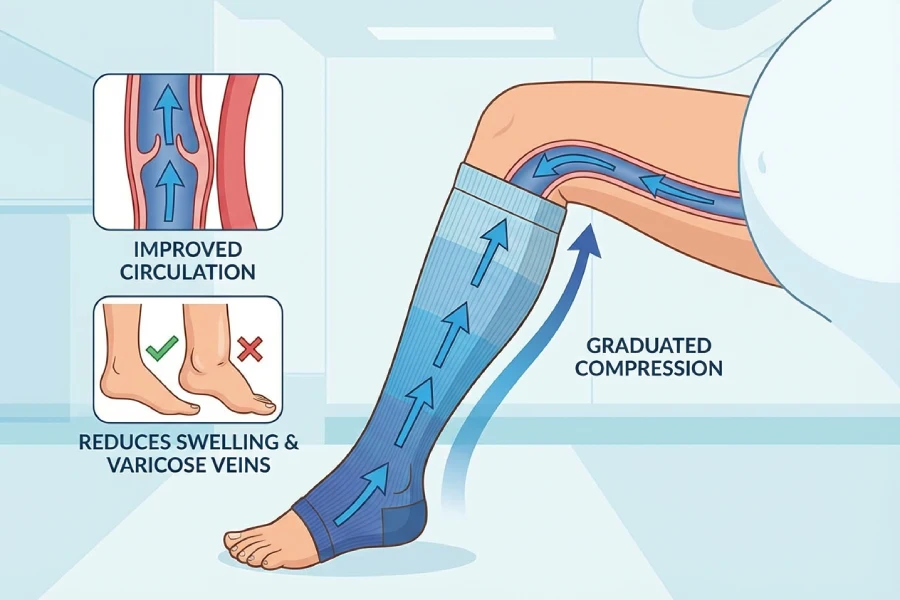
The Medical Case for Maternity Compression Socks
During pregnancy, the body produces roughly half again as much blood as before. Hormone levels change the way blood flows and clots. And the growing uterus presses on the pelvic veins can slow venous return from the legs. Many women also move less, especially late in pregnancy or after long working days. Together, these factors make the lower legs a risk zone.
The Best Compression Levels (mmHg) for Maternity Compression Socks
Graduated compression socks work on a simple idea. They apply higher pressure at the ankle and lower pressure higher up the leg. This pressure profile helps:
- Promote blood flow back to the heart
- Reduce leg and ankle swelling (edema)
- Prevent varicose veins and spider veins
- Lower the risk of Deep Vein Thrombosis (DVT)
You might be wondering… do pregnant women really use these every day? In many markets, doctors, midwives, and physiotherapists already recommend compression socks as part of routine pregnancy care. Research published in the BUMP found that wearing 15-20 mmHg compression stockings during pregnancy significantly reduced leg pain and improved the quality of life for pregnant women. For mild to moderate symptoms, a daily knee-high maternity compression sock in the right mmHg range can make a clear difference in comfort. For stronger symptoms or early varicose veins, higher compression can help, as long as a medical professional is involved. Also, for a deeper comparison, see our guide to 15–20 vs 20–30 mmHg compression socks.
Market Opportunity for Brands&Buyers
Pregnancy compression socks are not just a copy of standard compression socks. The numbers show a faster growth curve, driven by medical need and e-commerce demand.
The maternity compression socks market was valued at about $420 million in 2024 and is forecast to reach about $780 million by 2033, with a CAGR of 7.1%. That growth rate is higher than the general compression socks market, which grows around 4.1%, making maternity compression one of the fastest-expanding segments.
Market growth of pregnant compression socks
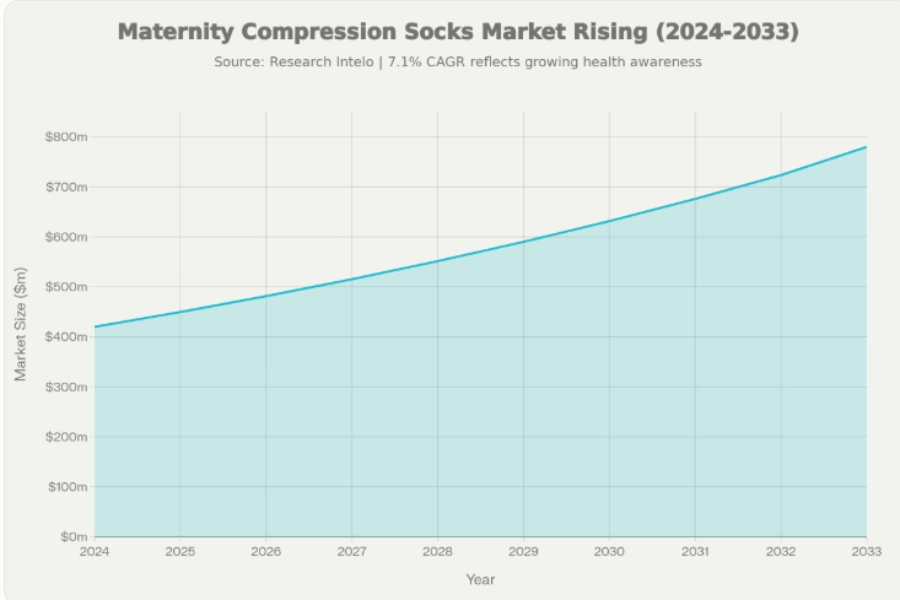
For buyers, the first question is simple: is this category worth building a product line around, or is it just a side item? The data points to a clear answer. Global reports place the 2024 maternity compression socks market in the range of $420–534.6 million. By 2033, this segment is forecast to almost double, reaching about $780 million and beyond.
Here’s the deal… this growth rate, around 7.1–7.2% CAGR, outpaces most other compression categories. General compression socks grow at about 4.1%. Medical compression apparel as a whole sits near 5%. Maternity compression socks are growing faster because they sit at the intersection of preventive health, pregnancy care, and lifestyle comfort.
Channel data matters for you as a buyer. Online stores already take about 36% of revenue and are the fastest-growing sales channel. Product type data also shows that graduated compression socks take about 57% of the maternity category. So if you build a line of graduated OEM maternity compression socks, you’re working with the core of the segment, not a side branch. By region, Asia Pacific shows the highest growth, with a projected CAGR of around 9.1–9.2%.
Key market drivers
- Healthcare awareness: Medical professionals increasingly recommend compression therapy during pregnancy
- Insurance coverage: In many countries, prescription compression socks qualify for insurance reimbursement
- E-commerce growth: Amazon and DTC maternity brands are expanding their compression sock offerings
- Fashion integration: Modern designs make compression socks acceptable for everyday wear
Leading maternity brands like Kindred Bravely, Comrad, and Motif Medical have demonstrated strong demand for quality maternity compression socks. For buyers, this creates opportunities to serve:
- Maternity-focused DTC brands
- Amazon private label sellers
- Healthcare distributors
- Pharmacy chains and drugstores
- Specialty retailers
Product Specifications for OEM Orders
Not all maternity compression socks are made in the same way. For Brands, small details in specs turn into big differences in returns and reviews.
Strong custom products use a nylon and spandex base, true graduated compression, and multiple sizes, including plus size and wide-calf. They also include reinforced heel and toe, soft top bands that don’t dig in, and moisture-wicking yarns that stay comfortable during long wear.

When sourcing custom compression socks OEM for the maternity market, consider these key specifications:
Compression Levels:
- 8-15 mmHg: Suitable for early pregnancy and mild symptoms, provides gentle support and a non-restrictive wearing feel
- 15-20 mmHg: Light compression for mild swelling, suitable for everyday wear
- 20-30 mmHg: Medium compression recommended by most healthcare providers
- 30-40 mmHg: Firm compression typically requiring a prescription
Materials:
- Nylon/Spandex blend (most common): Strength and color clarity, 70-90% nylon, 10-30% spandex
- Lycra®-certified materials: Premium option with verified elasticity
- Cotton blend: Breathable option for sensitive skin
- Copper-infused: Antimicrobial properties for extended wear
From an OEM manufacturing perspective, we see most maternity clients choosing bamboo and cotton-rich blends as their main base yarns because they offer a softer, more comfortable feel against the skin. On top of that, they often add copper-infused fibers or similar functional yarns to these bases to enhance performance, combining comfort with better hygiene and long-wear freshness.
Design Features:
- Graduated compression profile (tighter at ankle)
- Soft elastic band at top (non-restrictive)
- Reinforced heel and toe
- Moisture-wicking properties
- Multiple size options (S-XL, plus size)
Plus-size & wide-calf fit as a core spec:
Consider plus-size and wide-calf as core specs, not optional extras. Pregnancy-related swelling in the feet and lower legs means narrow socks are harder to pull on, dig into the calf, restrict circulation, and leave red, itchy marks. True plus-size and wide-calf maternity compression designs prevent this, keeping compression therapeutic instead of restrictive, reducing fit-related complaints and returns, and making your brand one of the few that truly fit changing pregnancy bodies.
Length Options:
- Knee-high: Most popular for daily wear
- Thigh-high: Maximum coverage for DVT prevention
- Ankle/crew: Casual option for mild symptoms
Certification Requirements
Maternity compression socks should be worn close to medical devices. Buyers in different regions expect clear compliance documentation, not just fabric quality claims.
For OEM maternity compression socks, look for support with FDA registration for the USA, CE marking and ISO 13485 for Europe, and global standards like OEKO-TEX for material safety and BSCI for social compliance— see our compression socks certifications for details.
| Market | Required Certifications |
|---|---|
| USA | FDA registration for medical devices, ASTM testing |
| EU | CE marking, ISO 13485 quality management |
| Global | OEKO-TEX for material safety, BSCI for ethical manufacturing |
Working with a manufacturer that maintains these certifications streamlines market entry and builds buyer confidence.
Customization Options for Brand Differentiation

Many brands still sell maternity compression socks in plain medical colors. That creates room for differentiation on your side. For a detailed breakdown of all available options, see our socks customization details.
- Branding: Custom packaging, hangtags, labels
- Colors and patterns: From medical neutrals to fashion-forward designs
- Size range: Extended sizing for plus-size pregnant women
- Specialty features: Open-toe vs. closed-toe, reinforced cushioning
- Packaging: Retail-ready, gift sets, subscription bundles
Most experienced manufacturers can provide free mockup designs within 24 hours, allowing brands to visualize products before sampling.
Selecting the Right Manufacturing Partner
When evaluating maternity compression sock manufacturers, consider a few key factors. For example, a factory like Max Hosiery checks boxes such as:
- Integrated manufacturing & export experience
In-house knitting and finishing plus independent import–export rights, with years of supplying compression and functional socks to North America and Europe. - Certifications and compliance
ISO9001 quality system, CE, BSCI, FDA, and OEKO-TEX Standard 100 to support medical, retail, and online channels. - Clear MOQs, samples, and lead times
Transparent minimum order quantities, a structured sampling process for custom and plus-size styles, and typical bulk production of around 30–45 days after sample approval. - Quality control and reliability
Defined QC at each stage, final inspection before shipment, and lab testing for colorfastness and dimensional stability so your products arrive retail-ready. - Design confidentiality
Willingness to sign NDAs and keep your branding, tech packs, and plus-size patterns strictly confidential.
Conclusion

The maternity compression socks market is growing fast, especially for brands that understand mmHg levels, plus-size fit, and certification needs. By partnering with an experienced OEM maternity compression socks manufacturer, you can launch reliable, comfortable products that meet the increasing demand for functional maternity wellness products.
Ready to develop your own OEM or wholesale pregnancy compression socks line? Contact our team for a free consultation and product catalog.
FAQ: OEM maternity compression socks for buyers
What compression levels should I include in my maternity compression socks line?
Most brands build their core range around 15–20 mmHg for daily use and add 20–30 mmHg styles for customers with more visible swelling or early varicose veins under medical guidance. Higher levels (30–40 mmHg) are usually prescription-only and not necessary for standard retail or e-commerce ranges.
Can you support plus-size and wide-calf maternity compression socks?
Yes. We can develop dedicated plus-size and wide-calf patterns with adjusted calf circumference and stretch distribution, not just longer lengths. You can offer a full-size run from standard S–XL plus extended sizes that truly fit changing pregnancy bodies.
What is the typical MOQ for OEM maternity compression socks?
MOQs depend on yarn type, design complexity, and number of colors/sizes, but we usually support flexible quantities per color and size for test orders. Share your channel plan (Amazon, pharmacy, DTC, etc.), and we’ll recommend a MOQ structure that balances cost and inventory risk.
How long does the sampling and production process take?
Digital mockups can usually be prepared quickly once we have your logo, colors, and specs. After you approve physical samples, bulk production for maternity compression socks typically takes around 30–45 days, depending on order volume and packaging.
Which certifications can you help with for maternity compression socks?
We can work according to requirements such as FDA support for the US market, CE/ISO for Europe, and OEKO-TEX/BSCI for material and social compliance. Let us know your target countries and sales channels so we can match the documentation and testing to your needs.
